MHSRS 2024 DAILY DIGEST – DAY 4
The fourth and final day of MHSRS concluded with sessions on the subjects of preventing infection with devices that treat battlefield wounds in prolonged care, clinical studies for advancing combat casualty care, managing fatigue and using AI in psychological disorders, exploring biomechanics of military injury, predicting and preventing future infectious disease outbreaks in military settings, treatments for shock and blood failure, leveraging tele-behavioral health to address shortages of behavioral health clinicians, preventing musculoskeletal injuries, and medical mobility and casualty evacuations in large scale combat operations. See below for summaries from the sessions and our discussions with military leadership.
HIGHLIGHTING HEROES
We’d like to give special recognition to these key life science innovators who are heroic and determined in their efforts to create dual use medical solutions and were invited to give podium or poster presentations during the conference:
- Humacyte is pioneering the development and manufacture of off-the-shelf, universally implantable, bioengineered human tissues. Their podium presentation highlighted clinical trial evaluation of safety and efficacy of their Acellular Tissue Engineered Vessels™ (ATEV™) to repair arterial traumatic injuries. Their work in helping patients in Ukraine is a badge of honor for the company and the DoD as they continually reference their leadership in that effort.
- American Type Culture Collection (ATCC) supports government research and development programs in global health, biomanufacturing, and infectious and chronic diseases. They gave two poster presentations: 1) extracellular vesicles for reversal of irradiation-induced cellular damage, and 2) rapid diagnosis of invasive fungal infections. The DoD research community uses ATCC’s repository as it is the world’s largest and most diverse collection of human and animal cell products and Assistant Secretary of Defense for Health Affairs Dr. Lester Martinez Lopez toured the facility last year, commenting on how increased engagement with DoD is key to biosecurity.
- Ampa offers precision brain stimulation using transcranial magnetic stimulation (TMS) with 50% efficacy in achieving full remission from depression in 5 days of treatment. Their poster presentation focused on extending access to TMS treatments for depression and PTSD in remote environments. Their handheld device was a big hit with some key leaders spanning from WRAIR to USAISR for Warfighters and Veterans.
Other innovators at the conference engaging military leadership to address military medical gaps include:
- Curavit provides a Decentralized Clinical Trial (DCT) platform that enrolls patients into clinical trials faster, with higher protocol adherence; resulting in better data quality, shorter timelines, lower costs and more diversity of subjects.
- Diagnostic Ventures provides a sepsis test with results in 1 hour or less, and per test cost is half of all other comparable options.
- Sonostik has a 510(k) cleared device expediting IV placement, assisting providers in advancing a catheter guide wire the moment the needle enters the vessel addressing the 40% prehospital fail rate of first-pass IV line placement in Warfighters due to complex life-threatening injuries characterized by traumatic hemorrhage and substantial tissue and organ damage.
Still others working to make a difference for the military and civilian populations, who piqued the interest of leaders the G2G talked to include: Celmatix, HealthyWomen, Medicines 360, NCBiotech, NeuroWave, United States Pharmacopeia (USP), and Women’s Health Access Matters (WHAM). With dual use for civilian applications a high priority for the Defense Health Agency that now manages all biomedical research and care for service members and their families, collaboration, flexibility, alignment, and patience are key. The government process for executing CRADAS, BAAs and legislative directives all take time, but can be accelerated through quality collaborations often first forged at MHSRS. We are so happy to help facilitate these connections and elevated profiles for some many critical innovations, companies and organizations.
CONFERENCE WRAP-UP
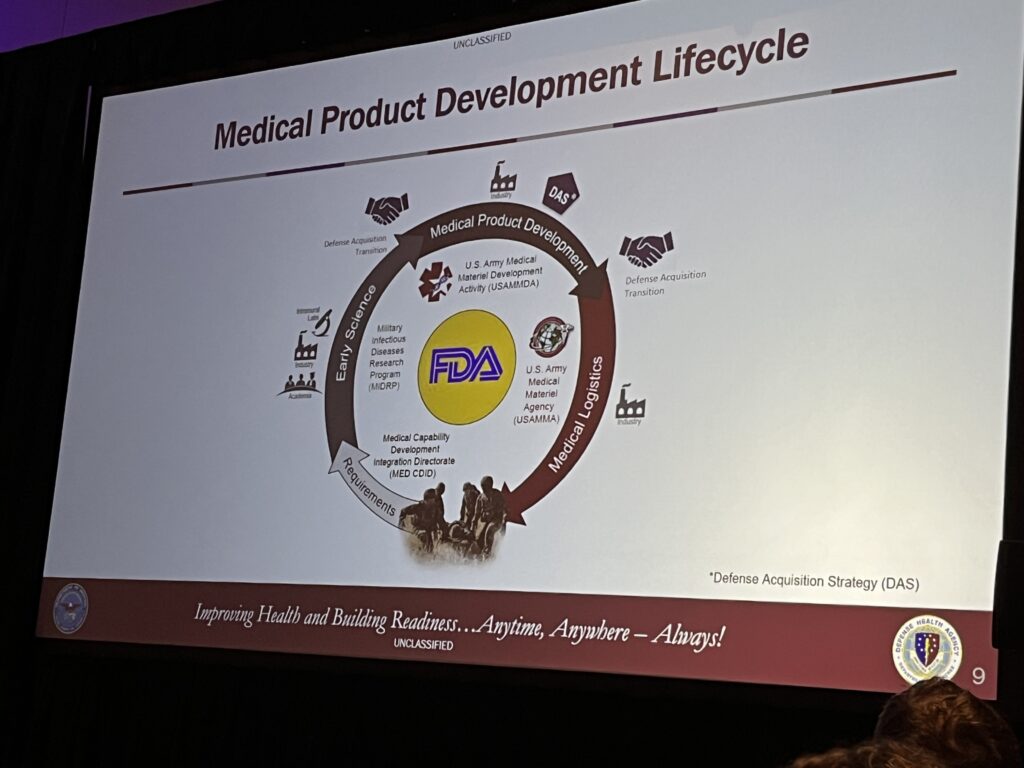
On the final day, the G2G focused on the U.S. Army Medical Materiel Development Activity (USAMMDA). USAMMDA’s mission is to protect and preserve the lives of U.S. military personnel through the development of new drugs, vaccines, devices, and medical support equipment that enhance readiness, ensure the provision of the highest quality medical care to the DOD, and maximize survival of medical casualties on the battlefield. Program Managers guide medical product development for the U.S. Army Medical Department, other U.S. Services, the Joint Staff, the Defense Health Agency, and U.S. Special Forces community. The medical product development lifecycle begins by taking requirements (medical gaps and needs determined to be priorities) from the Medical Capability Development Integration Directorate (MED CDID) and engaging U.S. Forces, industry, and academia in early science and testing required to move a product through the U.S. Food and Drug Administration (FDA) approval or licensing process in order to ultimately field and sustain finished products for the treatment, health and well-being of injured and sick active duty personnel and veterans.
USAMMDA has five Project Management Offices (PMO), two of which were highlighted in this morning’s session.
- Warfighter Expeditionary Medicine and Treatment (WEMT) Project Management Office focuses developing or modifying FDA-cleared medical devices to deliver new capabilities to the field for the Army and Joint Force in the following focus areas: noncompressible hemorrhage control, advanced medical monitoring, oxygen generation solutions (in the field), portable imaging systems, treatment of burns and wounds (in the battlefield), and sterilization in austere environments.
- Warfighter Protection and Acute Care (WPAC) Project Management Office aims to develop, deliver and field FDA-approved preventions, diagnostics, and treatments for infectious diseases and combat wound infections; blood products and blood components; and drugs for battlefield pain management. Current WPAC efforts focus on the development of vaccines, drugs, diagnostics, and therapeutics for the prevention and treatment of infectious diseases, hemorrhage control and resuscitation solutions for Warfighters and for Military working dogs, as well as drugs for battlefield pain management.
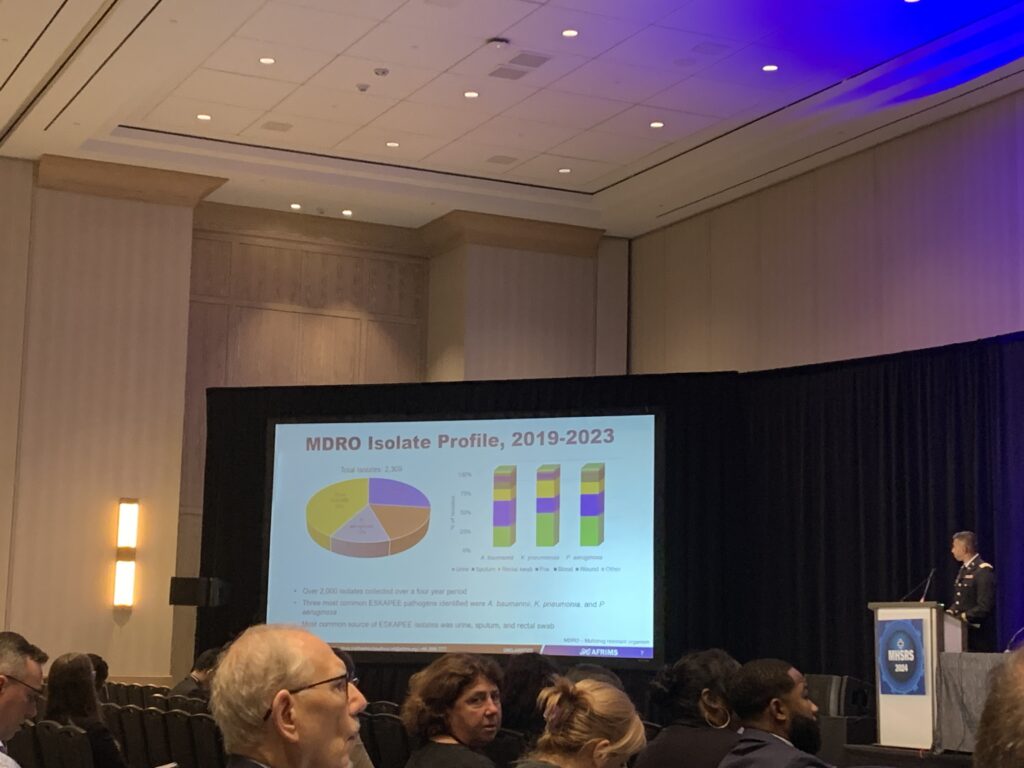
A special session on clinical studies on combat casualty care provided novel insights on the benefits of the pre-oxygenation of patients prior to emergency intubation, the targeted use of a novel opioid for alleviating pain from burn injuries, and miniaturizing vitals monitoring devices. Interestingly, a key study suggested the enhanced capacity of mobile health applications in diagnosing mild traumatic brain injury when compared to longstanding military concussion protocols. The sessions also highlighted the global footprint of military medical research – particularly in the emerging infectious diseases space. This includes the Armed Forces Research Institute of Medical Science based in Thailand, which completes large scale clinical trials for a range of disease countermeasures with a focus on licensure, as well as the US Army Medical Research Directorate-Africa based in Kenya, which completes trials for vaccines and therapeutics.
Other sessions true to the priorities set out by Assistant Secretary of Defense for Health Affairs Dr. Lester Martinez Lopez covered mental and behavioral health and lack of sleep. Sleep is a constant concern for warfighters knowing they tend to get significantly less sleep than needed but are expected to perform at peak performance at all times. Insomnia is prevalent, affecting 40-70% of warfighters, costs $1.8B a year, yet is treatable and can lead to better outcomes for the military. Research shows 24 hours of sleep loss or 5 consecutive days of 5 hours of sleep leaves you functioning as though you are legally intoxicated. Research on why sleep is affected for warfighters and what therapeutics and technologies may enhance sleep were highlighted.
THANK YOU
As we wrap up the week of MHSRS, we would like to thank those who joined us this week and those we got to represent and of course, all those who have served in the military. G2G is truly honored to work with all of you! We look forward to building traction made this year and will start planning our annual webinar on prepping for MHSRS with abstract submissions in February 2025. Stay tuned!